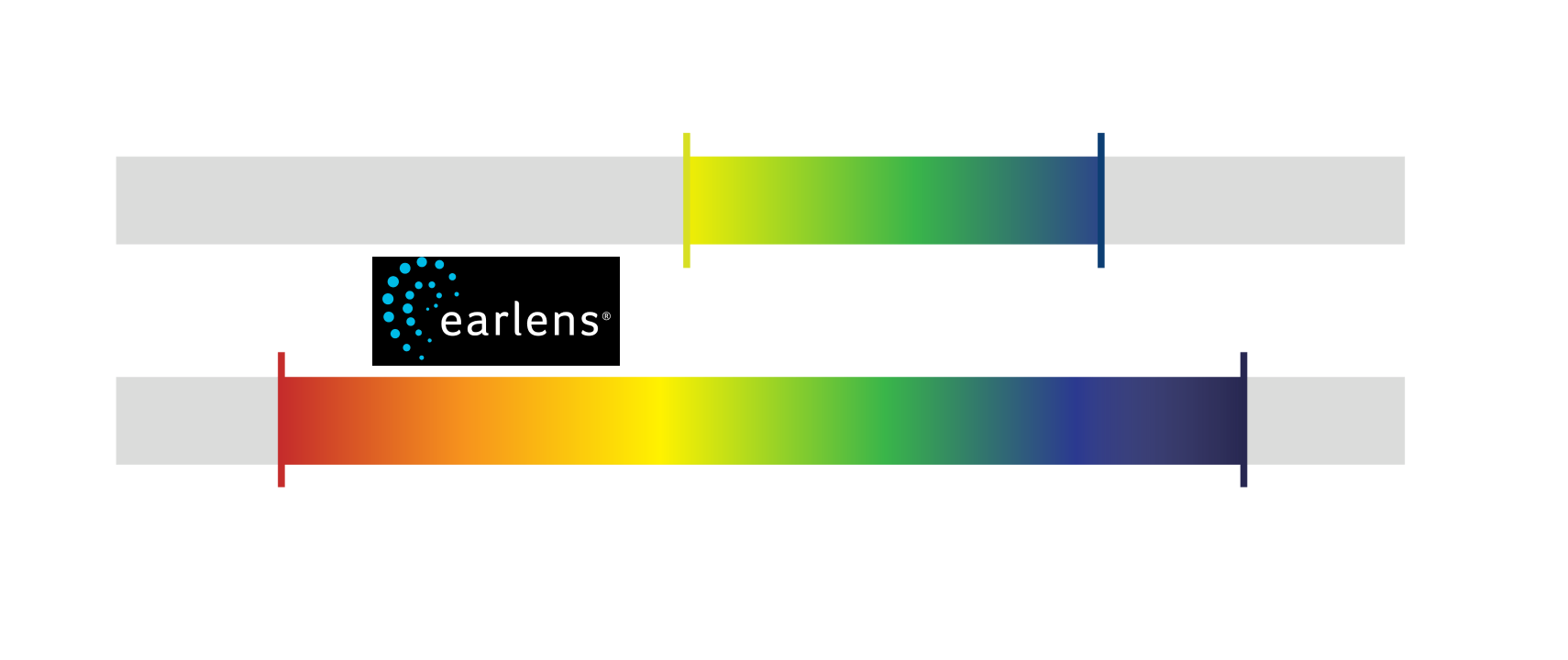
Earlens Physicians

"This is the only technology that I offer where patients routinely cry tears of joy when we activate them. So it's very gratifying for me to be able to offer something like this to my patients."
Dr. Keith Matheny
See More Stories